Lustran® ABS 248FC
| 牌号 | Lustran® ABS 248FC |
| 厂家 | INEOS ABS (USA) |
| 分类 | ABS |
| 介绍 | Lustran ABS 248FC resin is a high-gloss, medium-impact grade of ABS (acrylonitrile butadiene styrene). This injection molding grade offers a good balance of physical properties and has been designed to be compliant with both medical and food contact regulations. Lustran ABS 248FC resin is used in applications requiring rigidity and intermediate abuse resistance. As with any product, use of Lustran ABS 248FC resin in a given application must be tested (including field testing, etc.) in advance by the user to determine suitability. Lustran ABS 248FC 000000 (natural) complies with FDA regulation 21 CFR 181.32 for repeated-use food-contact applications. It is also compliant with EU Directive 2002/72/EC and its amendments (2004/1/EC, 2004/19/EC, 2005/79/EC, 2008/39/EC) relating to plastic materials and articles intended to come into contact with foodstuffs. Lustran ABS 248FC resin is designated as "medical-grade" and has met the requirements of the USP Class VI and ISO 10993, Part I "Biological Evaluation of Medical Devices" tests with human tissue contact time of 30 days or less. Only medical-grade resins may be considered as candidates for applications requiring biocompatibility. . Regrind must not be used in medical applications requiring biocompatibility. |
| INEOS ABS (USA) 产品展示 | 种类 |
|---|---|
| Lustran® ABS 556 | ABS |
| Lustran® ABS 552 | ABS |
| Lustran® ABS 266 | ABS |
| Lustran® ABS 682 | ABS |
| Lustran® ABS 633 | ABS |
| Lustran® ABS Guardian™ 620 | ABS |
| Lustran® ABS 256 | ABS |
| Lustran® ABS 752 Q374 | ABS |
| Lustran® ABS 446 | ABS |
| Lustran® ABS 248FC | ABS |
订购说明
上海松翰塑化科技有限公司订货流程
【订购导引】
我司所销售所有产品均为原厂原包,均可提供相关资料报告(如:物性表,MSDS报告,UL报告,COA报告,SGS报告,FDA等)!
如您需要提前了解原料产品的相关性能或是需要原料产品的相关报告,请您通过电话或是客服QQ与我联系!
【物流说明】
自接到贵司订购单24小时内发货,珠三角,长三角,北京,天津均支持代收货款。货物送达后,请第一时间检查外包装,如发现物流过程中出现大量包装损坏及原料外漏的,请让物流人员出具证明并保存,并在24小时内与我司客服部取得联系。
【订购流程】
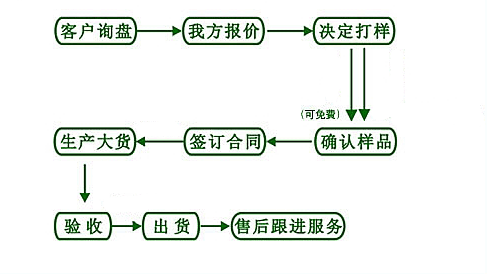
【退换货处理】
退货:
依照中华人民共和国消费者保护法规,在您收到货品7天内,若发现您收到的商品有瑕疵或损坏,您可以要求退货,请主动与本公
司联络,敝公司接到您的商品之后,会将您的购物金额全额退回。
换货:
若您收到的商品有瑕疵或损坏,请您于7天内与本公司联络,本公司有专人处理换货事宜。
我司所销售所有产品均为原厂原包,均可提供相关资料报告(如:物性表,MSDS报告,UL报告,COA报告,SGS报告,FDA等)!
如您需要提前了解原料产品的相关性能或是需要原料产品的相关报告,请您通过电话或是客服QQ与我联系!
【物流说明】
自接到贵司订购单24小时内发货,珠三角,长三角,北京,天津均支持代收货款。货物送达后,请第一时间检查外包装,如发现物流过程中出现大量包装损坏及原料外漏的,请让物流人员出具证明并保存,并在24小时内与我司客服部取得联系。
【订购流程】

【退换货处理】
退货:
依照中华人民共和国消费者保护法规,在您收到货品7天内,若发现您收到的商品有瑕疵或损坏,您可以要求退货,请主动与本公
司联络,敝公司接到您的商品之后,会将您的购物金额全额退回。
换货:
若您收到的商品有瑕疵或损坏,请您于7天内与本公司联络,本公司有专人处理换货事宜。


